Two distinct tetranuclear motifs, rectangular vs. extended, are observed for complexes of a bis-tetradentate pyrimidine-based ligand†
Abstract
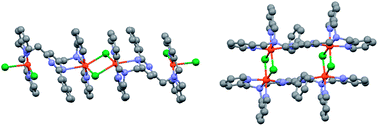
The 1 : 2 reactions of 4,6-bis[N,N-bis(2′-pyridylmethyl)aminomethyl]-2-phenylpyrimidine (LMe) and MnII, CoII, NiII and CuII chloride salts in MeOH gave the tetranuclear complexes [MnII4(LMe)2Cl8]·2H2O (1), CoII4(LMe)2Cl8·10H2O (2), [Ni4II(LMe)2Cl4]Cl4·8H2O (3) and [CuII4(LMe)2Cl6]Cl2·8H2O (4), respectively. X-ray crystal structure determinations carried out on three of the complexes revealed that in the case of NiII (3′) the LMe ligand is bound in the expected bis-tetradentate fashion, bridging the encapsulated pair of NiII centers via the pyrimidine ring, and that two pairs of chloride anions bridge a pair of such dimetallic units, affording a closed rectangular tetranuclear core overall. In contrast, in the case of MnII (1′) and CuII (4′) only one binding pocket of the LMe ligand strand binds in the expected tetradentate manner, whilst the other binds in a terdentate manner as one pyrimidine nitrogen atom does not bind, so in these cases the pyrimidine moiety does not bridge the metal centres, resulting instead in ‘extended’, linear tetranuclear assemblies.

 Please wait while we load your content...
Please wait while we load your content...