Synthesis of Markovnikov vinyl sulfides via dinuclear Rh(i)-phosphine catalyzed hydrothiolation of alkynes in aqueous media†
Abstract
An efficient and green procedure for the synthesis of Markovnikov i.e. branched vinyl sulfides in aqueous media was developed by employing the dinuclear Rh(I) complexes of hydrophilic phosphines as catalysts in the alkyne hydrothiolation with aliphatic thiols. Deuterium-labeling studies provide evidence for the aptness of these dinuclear catalysts to form selectively the Markovnikov syn-addition products. Catalyst order experiments disclose that the order with respect to the concentration of the catalyst is one, i.e. in solution the active catalyst is dinuclear with one active metal center and the second metal center contribute the cooperative effects to influence the Markovnikov selectivity in hydrothiolation. Further, the possibility of catalyst recovery and recycling experiments were also demonstrated.
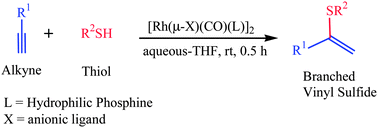

 Please wait while we load your content...
Please wait while we load your content...