Palladium catalyzed intermolecular hydroamination of 1-substituted allenes: an atom-economical method for the synthesis of N-allylamines
Abstract
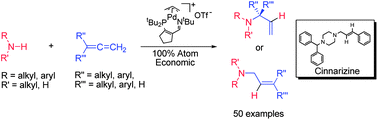
The palladium complex [(3IPtBu)Pd(allyl)]OTf previously displayed excellent catalytic activity for the hydroamination of 1,1-dimethylallene with anilines, selectively producing the branched substituted allylamine product (kinetic product) in high conversion. In the current report, the scope of this hydroamination reaction has been expanded to include both alkyl amines and anilines in combination with an array of seven alkyl and aryl allenes. For the majority of amines investigated, the hydroamination of 1,1-dimethylallene, cyclohexylallene, benzylallene, and select aryl allenes with alkyl amines gave the branched substituted allylamine product in nearly quantitative conversion at ambient temperature in less than 1 hour. In contrast, anilines displayed a more limited reaction scope and yielded the linear hydroamination product (thermodynamic product) with all allenes other than 1,1-dimethylallene. Both branched and linear products could be formed selectively in the hydroamination of p-fluorophenylallene with alkyl amines through careful control of [(3IPtBu)Pd(allyl)]OTf catalyst loading and reaction duration. Overall, the branched allylamines produced are useful synthetic intermediates due to their available unsaturated vinyl group, while the linear allylamine products are chemically similar to a class of known pharmaceuticals.

 Please wait while we load your content...
Please wait while we load your content...