Mechanism for H2 release from potential hydrogen storage materials of phosphine alane and phosphine borane in the presence or absence of alane or borane: a theoretical study†
Abstract
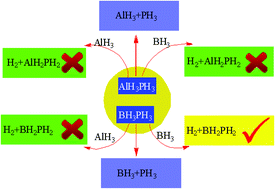
Electronic structure calculations have been employed to probe the reaction mechanisms of hydrogen release from phosphine alane and phosphine borane in the presence or absence of alane and borane. Geometries of stationary points are optimized at the MP2/aug-cc-pVDZ level. Then, the energy profiles are refined at the CCSD(T)/aug-cc-pVTZ level based on the MP2 optimized geometries. In both AlH3PH3 and BH3PH3 monomer, H2 elimination cannot be compared with the Al–P and B–P bonds dissociation. The theoretical results demonstrate that both AlH3 and BH3 can facilitate H2 dissociation from AlH3PH3. However, the H2 production process becomes more difficult once the strong adduct is formed. Similarly, AlH3 and BH3 can also take part in a catalytic process for H2 loss from BH3PH3 and induce a substantial reduction of the energy barrier for H2 release. Comparison with the reaction mechanisms of H2 elimination from BH3PH3 with AlH3 or BH3 as the catalyst shows that BH3 is a better catalyst than AlH3. Moreover, BH3PH3 is more favored as a potential hydrogen storage material than AlH3PH3 in the presence of AlH3 or BH3. So a catalyst is required as the generation of H2 from phosphorous systems proceeds with an energy barrier much higher than the X–P (X = Al and B) bond energy.

 Please wait while we load your content...
Please wait while we load your content...