Triflic acid mediated functionalization of α-hydroxyphosphonates: route for sulfonamide phosphonates†
Abstract
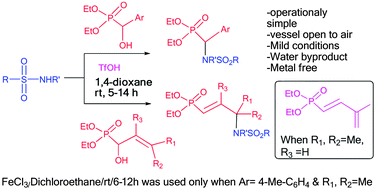
An operationally simple synthetic method for (±)-α-aryl/methylsulfonamidomethylphosphonates and new (±)-γ-aryl/methyl sulfonamidomethylvinylphosphonates has been developed through straightforward reactions of (±)-α-hydroxyphosphonates with sulfonamides in the presence of triflic acid (TfOH) at room temperature in a vessel open to air. For γ-dimethylallylhydroxyphosphonate, the (E)-1,3-butadienylphosphonate was formed quantitatively using TfOH while FeCl3 afforded the expected product in moderate yield unpredictably. The favourable sulfonoamidation of benzyl alcohol is also observed when TfOH was used for α-hydroxyphosphonates having a benzyloxy group.

 Please wait while we load your content...
Please wait while we load your content...