Nitro ⇌ aci-nitro tautomerism and E/Z isomeric preferences of nitroethenediamine derivatives: a quantum chemical study†
Abstract
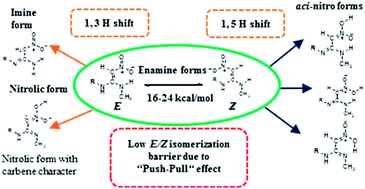
The nitroethenediamine moiety is present in several important chemical species including therapeutic agents like ranitidine. In the scientific literature, the ranitidine molecule is represented in three different tautomeric forms: enamine, imine and nitronic acid (aci-nitro) and the observed polymorphic differences in ranitidine are attributed to the structural differences. Quantum chemical calculations have been performed using HF, B3LYP, BHandHLYP, M06-2X, and MP2 methods, on model nitroethenediamine to understand the isomeric preferences. Calculations indicated that in model nitroethenediamine, enamine is the most preferred tautomeric state which exists in two geometrical isomers (E/Z). These isomers are in dynamic equilibrium due to low barriers (∼14 kcal mol−1 in solvent conditions) for rotation across the C1–C2 double bond as a result of a push–pull effect of the nitroethenediamine moiety. The aci-nitro form of the nitroethenediamines is invoked in explaining the polymorphism and metabolism as well as the activity of these species. The relative importance of the aci-nitro form is explored in this article. The results indicate that the aci-nitro form becomes easily accessible under acidic conditions, though this tautomer is about 13 kcal mol−1 less stable under the gas phase conditions. The observed polymorphic differences have been traced to easy E/Z conversion and the tautomerism under acidic conditions.

 Please wait while we load your content...
Please wait while we load your content...