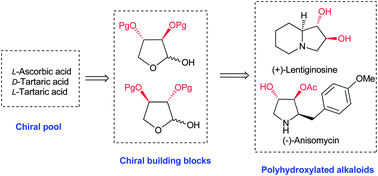
Practical synthesis of trans-dihydroxybutyrolactols as chiral C4 building blocks and their application to the synthesis of polyhydroxylated alkaloids†
Abstract
Practical syntheses of trans-dihydroxybutyrolactols 2a, 2b and 2c from inexpensive chiral pool compounds L-ascorbic, D- and

 Please wait while we load your content...
Please wait while we load your content...