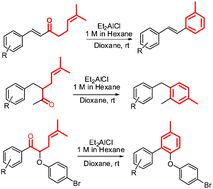
Transition metal-free domino sequential synthesis of (E)-stilbenes, biaryl methanes and biaryl ethers using Et2AlCl as a Lewis acid†
Abstract
A transition metal-free domino process has been developed, for the first time, to synthesize (E)-stilbenes, biaryl methanes and biaryl

 Please wait while we load your content...
Please wait while we load your content...