Amphiphilic photocleavable block copolymers based on monomethyl poly(ethylene glycol) and poly(4-substituted-ε-caprolactone): synthesis, characterization, and cellular uptake
Abstract
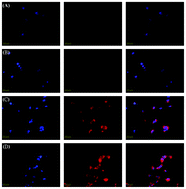
This paper presents the synthesis of a novel amphiphilic block copolymer containing photodegradable linkers as junction points between hydrophilic and hydrophobic chains. MPEG-ONB-PXCL block copolymers were synthesized by a combination of ROP and “clicks” from a difunctional photoresponsive initiator (ONB). The copolymers are biodegradable and biocompatible, and can self-assemble into photoresponsive micelles. When polymer solutions were exposed to UV, we observed significant changes in the structure and morphology of particles. Fluorescence emission measurements showed that Nile red (NR), a hydrophobic dye, encapsulated by the MPEG-ONB-PXCL micelles, is released upon irradiation because of disruption of the micelles. Light-triggered bursts were observed for Indomethacin (IMC)-loaded MPEG-ONB-PXCL micelles upon at 30 min of light irradiation. No significant toxicity of these nanoparticles was found, at concentrations up to 1000 μg mL−1, before or after light irradiation. The doxorubicin (DOX)-loaded micelles facilitated improved uptake of DOX by HeLa cells within 60 min, and were retained primarily in the cytoplasm.

 Please wait while we load your content...
Please wait while we load your content...