Reduced graphene oxide induced confined growth of PbTe crystals and enhanced electrochemical Li-storage properties†
Abstract
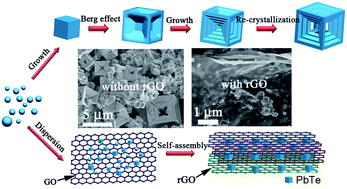
PbTe/reduced-graphene-oxide (PbTe/rGO) nanohybrids have been synthesized by a facile in situ solvothermal method using graphite oxide, Pb(NO3)2, Na2TeO3, and NaOH as the raw materials. The growth of PbTe and the reduction of graphene oxide occur simultaneously to form the PbTe/G hybrids composed of few-layer rGO and PbTe submicron crystals (100–200 nm). The presence of rGO inhibits the crystal growth of PbTe, decreasing the crystal size of PbTe from the microscale to submicron size. The PbTe/rGO hybrid with an appropriate amount of rGO exhibits remarkably improved Li-storage properties compared with bare PbTe, due to the unique hybrid structure, small size of the PbTe, and the combined conducting, buffering and confining effects of the rGO.

 Please wait while we load your content...
Please wait while we load your content...