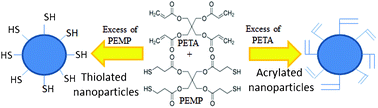
Synthesis of thiolated and acrylated nanoparticles using thiol-ene click chemistry: towards novel mucoadhesive materials for drug delivery†
Abstract
* Corresponding authors
a Rīga Stradiņš University, Faculty of Pharmacy, Riga, LV-1007, Latvia
b
Reading School of Pharmacy, University of Reading, Reading, United Kingdom
E-mail:
v.khutoryanskiy@reading.ac.uk
Fax: +44(0)1183784703
Tel: +44(0)118378669

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
A. Štorha, E. A. Mun and V. V. Khutoryanskiy, RSC Adv., 2013, 3, 12275 DOI: 10.1039/C3RA42093K
This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence. You can use material from this article in other publications, without requesting further permission from the RSC, provided that the correct acknowledgement is given and it is not used for commercial purposes.
To request permission to reproduce material from this article in a commercial publication, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party commercial publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
