d-Fructose-derived β-amino alcohol catalyzed direct asymmetric aldol reaction in the presence of p-nitrophenol†
Abstract
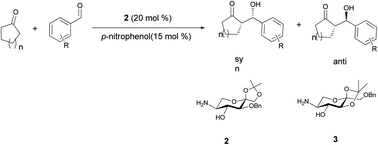
D-Fructose derived β-amino alcohols 2 and 3 were used as organocatalysts for direct asymmetric aldol reaction of various aromatic aldehydes with three kinds of cyclic ketones in the presence of different additives as a co-catalyst. The results showed that the combinations of β-amino alcohol 2 and p-nitrophenol built up a novel catalytic system. Loading of 20 mol% 2 and 15 mol% p-nitrophenol gave excellent yields (up to 98% with respect to aldehyde) of aldol reaction products with good enantioselectivity (up to 87% ee). Accordingly, a mechanism for the reaction was proposed by 1H NMR spectrum in this paper. Furthermore, the catalysts can be reused and have the significant catalyst recovery (77–84%).

 Please wait while we load your content...
Please wait while we load your content...