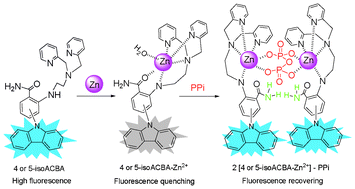
A new mononuclear Zn(II) complex based receptor for PPi sensing and alkaline phosphatase assay was fabricated using 2-aminobenzamide as a scaffold, appended with N,N-bis(2-pyridylmethyl) ethylenediamine (BPEA) and amide as a chelator and carbazole as a fluorophore. Based on an “ON–OFF–ON” molecular switch with zinc ion and PPi inputs, two highly efficient fluorescent chemosensors (4- and 5-isoACBA) were developed, which show a large binding affinity (Ka ≈ 2.5 × 1010 M−2) and high selectivity for PPi (43–48 fold fluorescent enhancement) over a wide range of anions including Pi, AMP, ADP and ATP in an aqueous HEPES buffer (pH 7.4). The binding mode of isoACBA–Zn(II) in the absence or presence of PPi was thoroughly investigated by UV and fluorescence titrations, ESI-HRMS analysis, and DFT studies. The results reveal that the synergistic effects of metal coordination and hydrogen bonding interactions in the structures of the isoACBAs should contribute to the remarkable binding affinity and high sensing selectivity for PPi.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...