An exclusive approach to 3,4-disubstituted cyclopentenes and alkylidene cyclopentenes via the palladium catalyzed ring opening of azabicyclic olefins with aryl halides†
Abstract
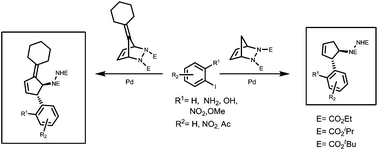
A simple and efficient method for the stereoselective ring opening of bicyclic

 Please wait while we load your content...
Please wait while we load your content...