Mild and convenient one-pot synthesis of 2-amino-1,3,4-oxadiazoles promoted by trimethylsilyl isothiocyanate (TMSNCS)†
Abstract
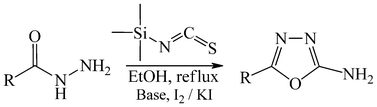
A mild, convenient, and efficient one-pot synthesis of amino-1,3,4-oxadiazoles is described. In situ preparation of various thiosemicarbazides by the reaction of different carboxylic acid hydrazides with

 Please wait while we load your content...
Please wait while we load your content...