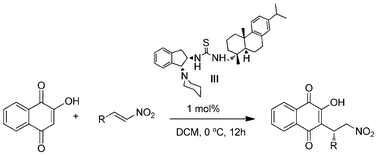
Enantioselective Michael addition of 2-hydroxy-1,4-naphthoquinone and 1,3-dicarbonyls to β-nitroalkenes catalyzed by a novel bifunctional rosin-indane amine thiourea catalyst†
Abstract
A novel bifunctional rosin-derived indane amine thiourea organocatalyst has been prepared and evaluated its catalytic efficiency in enantioselective

 Please wait while we load your content...
Please wait while we load your content...