Triton-X-100 catalyzed synthesis of 1,4-dihydropyridines and their aromatization to pyridines and a new one pot synthesis of pyridines using visible light in aqueous media†
Abstract
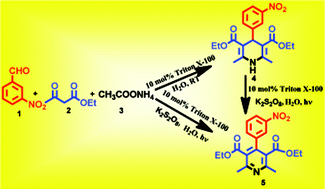
A realistic and convenient synthetic method has been developed for the facile synthesis of 1,4-dihydropyridine derivatives in the presence of the non-ionic surfactant Triton X-100, in an aqueous medium at room temperature. A greener method to synthesize pyridine derivatives has also been developed by the oxidation of 1,4-dihydropyridine derivatives with almost 100% yields and also in a one pot synthesis, employing an aldehyde, ethyl acetoacetate and ammonium acetate in an aqueous micellar medium by irradiation with potassium persulphate in the presence of visible light. The one pot protocol offered excellent yields of the targeted product in a very short period of time at room temperature and the non-ionic surfactant catalyst can be recovered very easily. We also observed that during the reaction there was the formation of micelles, or micelle-like colloidal aggregates, from the non-ionic surfactant and the reaction mixture in water, measured by dynamic light scattering and visualized through an optical microscope. The process is advantageous as ammonia is generated from an ammonium salt under absolutely neutral conditions and the product purification follows a group assistant purification chemistry process (GAP).

 Please wait while we load your content...
Please wait while we load your content...