Thiol–ene coupling kinetics of d-limonene: a versatile ‘non-click’ free-radical reaction involving a natural terpene†
Abstract
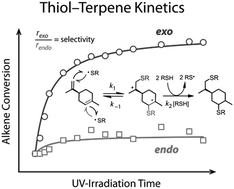
The free-radical photoinduced
* Corresponding authors
a Department of Fibre and Polymer Technology, School of Chemical Science and Engineering, KTH Royal Institute of Technology, SE-100 44 Stockholm, Sweden
b
Department of Chemistry, School of Chemical Science and Engineering, KTH Royal Institute of Technology, SE-100 44 Stockholm, Sweden
E-mail:
matskg@kth.se
Tel: +46-8-790 92 87
The free-radical photoinduced

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
M. Claudino, M. Jonsson and M. Johansson, RSC Adv., 2013, 3, 11021 DOI: 10.1039/C3RA40696B
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
