New asymmetric approach to β-trifluoromethyl isoserines†
Abstract
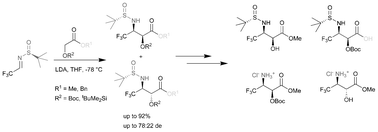
Enantiomerically pure N,O-protected β-trifluoromethyl isoserine derivatives of (2S,3S)- and (2R,3S)-absolute configurations have been easily prepared by diastereoselective addition of the enolates, derived from O-protected α-hydroxyacetates, to (S)-N-tert-butanesulfinyl (3,3,3)-trifluoroacetaldimine with high combined yield and good syn/anti stereoselectivity. To explain the unusual stereochemical outcome in these reactions a mechanistic rationale involving the addition of Z-enolates to (S)-imines via open transition states was proposed on the basis of the experimental data. Elaboration of these products via chemoselective manipulation of the protecting groups has been demonstrated.

 Please wait while we load your content...
Please wait while we load your content...