Three-component domino reactions providing rapid and efficient routes to fully substituted pyrroles†
Abstract
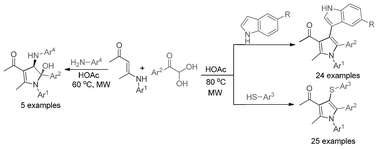
New indolation and thiolation-based three-component domino reactions, promoted by Brønsted acid, have been described, and 49 examples of polyfunctionalized fully substituted

 Please wait while we load your content...
Please wait while we load your content...