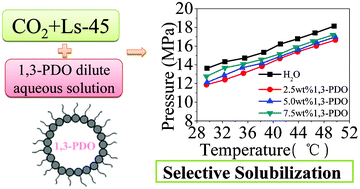
In order to purify the fermentation broth efficiently, this paper focuses on solubilizing polyalcohols from their dilute aqueous solution using a supercritical CO2 microemulsion (W–C microemulsion). The phase behavior of four systems was studied, including Ls-45/CO2, Ls-45/CO2/H2O, Ls-45/CO2/1,3-propanediol (1,3-PDO), and Ls-45/CO2/H2O/1,3-PDO, with 1,3-PDO dilute aqueous solution used to simulate the real fermentation broth, supercritical CO2 (scCO2) as the continuous phase, and the non-fluorous and non-silicon non-ionic Ls-45 as surfactant. The effects of Ls-45 concentration and 1,3-PDO concentration on the selective solubilization of 1,3-PDO in the Ls-45/CO2/1,3-PDO/H2O quaternary system are discussed at temperatures of 29.5–49.9 °C and at pressures of 7.22–19.27 MPa. The results show that a thermodynamically stable microemulsion can be formed by controlling the operating pressure and temperature and can selectively solubilize 1,3-PDO from its dilute aqueous solution. Both the Ls-45 concentration and 1,3-PDO concentration in the system have great influence on the selectivity of solubilzing 1,3-PDO from dilute aqueous solution. Moreover, it is found that the addition of ethanol (EtOH) can significantly decrease the operating pressure of forming the W–C microemulsion system. This result is useful for further research on the selective extraction of polyalcohols in biotechnology and helpful for industrialization.

 Please wait while we load your content...
Please wait while we load your content...