Synthesis of coumarins via PIDA/I2-mediated oxidative cyclization of substituted phenylacrylic acids†
Abstract
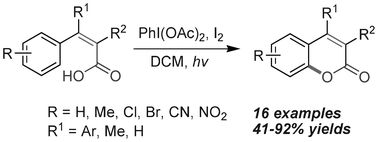
A variety of functionalized coumarins were synthesized from substituted phenylacrylic acids via PIDA/I2-mediated and irradiation-promoted oxidative carbon–oxygen bond formation. Our studies show that the oxygen in the pendant carboxylic acid group cyclizes favorably to the aryl ring that is cis to it. The main advantages of this method include good functional group tolerance and the transition-metal-free characteristic.

 Please wait while we load your content...
Please wait while we load your content...