An iterative, facile stereoselective synthesis of C1-C11 fragment of borrelidin via enzymatic desymmetrization strategy†
Abstract
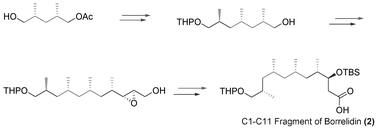
A highly stereoselective and general method for the synthesis of the C1-C11 fragment of borrelidin has been achieved. The main feature of our synthetic route is enzymatic desymmetrization to create two

 Please wait while we load your content...
Please wait while we load your content...