Photodecomposition of N-hydroxyurea in argon matrices. FTIR and theoretical studies†
Abstract
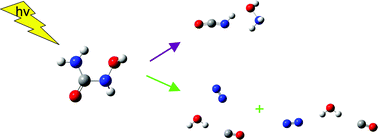
The photochemistry of N-hydroxyurea in solid argon has been investigated by FTIR and ab initio calculations. The irradiation of the NH2CONHOH/Ar matrices with the full output of the Xe arc lamp leads to the formation of the HNCO–NH2OH and N2–H2O–CO complexes. For the isocyanic acid–hydroxylamine complex, the spectra prove the existence of the hydrogen bonded structure with the NH group of HNCO attached to the oxygen atom of the NH2OH molecule. Two structures were identified for the nitrogen–water–carbon monoxide complex. In the first one, water is hydrogen bonded to the carbon atom and interacts with the nitrogen atom through van der Waals forces. In the second structure, water serves as a proton donor toward the nitrogen and carbon atoms of N2 and CO molecules, respectively. The identification of the products is confirmed by deuterium substitution and by MP2 calculations of the structure and vibrational spectra of the identified complexes.

 Please wait while we load your content...
Please wait while we load your content...