Effect of Zr substitution for Ce in BaCe0.8Gd0.15Pr0.05O3−δ on the chemical stability in CO2 and water, and electrical conductivity†
Abstract
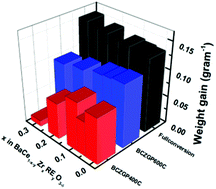
In this paper, for the first time, we report the chemical stability of a highly proton conducting Gd+Pr-codoped BaCe0.8−xZrxGd0.15Pr0.05O3−δ (BCZGP) (0.01 < x < 0.3) as a function of Zr-doping in H2O vapour, 30 ppm H2S in H2, and pure CO2 along with its electrical conductivity in air, N2 + 3% H2O, H2 + 3% H2O and N2 + D2O. All prepared BCZGP compositions retain the original cubic perovskite-type structure in 30 ppm H2S in H2 at 600 °C. BCZGP with x = 0.3 shows significant stability under pure CO2 at 400 °C, while upon exposure to H2O vapor all compositions form Ba(OH)2·xH2O. The maximum electrical conductivity obtained with higher Zr-doping in BCZGP (x = 0.3) is 7.6 × 10−3 S cm−1 which is about 30% of that of the parent compound BaCe0.8Gd0.15Pr0.05O3−δ. Current work clearly shows that Zr-doping at x = 0.3 increases the stability of BCZGP under 30 ppm H2S and pure CO2 at intermediate temperatures (T ≤ 400 °C), and retains good proton conductivity in H2 containing atmosphere.

 Please wait while we load your content...
Please wait while we load your content...