A coal desulfurization process via sodium metaborate electroreduction with pulse voltage using a boron-doped diamond thin film electrode†
Abstract
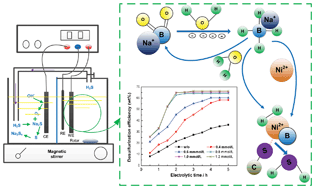
A preliminary study was conducted on the coal desulfurization process via NaBO2 electroreduction with pulse voltage using a boron-doped diamond (BDD) thin film electrode. It has been proved that NaBO2 was converted into NaBH4 by 11B NMR. The factors that influence the conversion rate of NaBO2 into NaBH4 and coal desulfurization efficiency were investigated. Under the conditions of −1.5 V forward pulse voltage, +0.5 V reverse pulse voltage, 0.2 mol L−1 NaBO2 concentration, 0.5 mol L−1 NaOH concentration, 2 s forward pulse duration, 1 s reverse pulse duration, 50 g L−1 coal concentration, 0.8 mmol L−1 NiCl2 concentration and 2.5 h electrolytic time, a higher desulfurization efficiency (64%) was obtained compared with the common electrochemical desulfurization (ECDS) process using a Pt electrode. By analyzing and comparing the coal samples and electrolytes before and after desulfurization it was indicated that the removed S from the coal sample in the form of gaseous H2S was mainly converted into Na2S and Na2Sx and boron (B) recycling was realized during coal desulfurization. Particularly, the combustion characteristics of coal were improved after desulfurization. Finally, the desulfurization mechanism was proposed. All these results indicated that the desulfurization process via NaBO2 electroreduction with pulse voltage using a BDD thin film electrode is effective and highly promising for coal desulfurization.

 Please wait while we load your content...
Please wait while we load your content...