Immobilization of MacMillan catalyst via controlled radical polymerization: catalytic activity and reuse†
Abstract
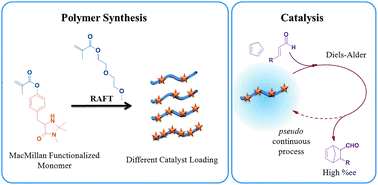
The MacMillan catalyst is an established organocatalyst capable of catalyzing a variety of organic reactions. Through the synthesis of a novel monomer containing the MacMillan catalytic functionality, a variety of copolymers have been synthesized with the comonomer, diethylene glycol methyl ether methacrylate (DEGMA). Reversible addition–fragmentation chain transfer (RAFT) polymerization was used for the synthesis of these functional polymers with good control over molecular weight, catalyst incorporation and polydispersity. These polymers showed lower critical solution temperature (LCST) behaviour where the cloud point was found to be dependent upon the degree of catalyst incorporation and catalyst loading was also found to have an effect on the Tg of the copolymers. The catalytic activity of the functional copolymers is demonstrated by the Diels–Alder reaction between cyclopentadiene and trans-hexen-1-al and shows enantioselectivity close to those previously reported by MacMillan. The polymers can be reused in multiple Diels–Alder reactions via a pseudo continuous process, maintaining high conversion and enantioselectivity throughout the cycles.

 Please wait while we load your content...
Please wait while we load your content...