Biocompatible polyethylenimine-graft-dextran catiomer for highly efficient gene delivery assisted by a nuclear targeting ligand†
Abstract
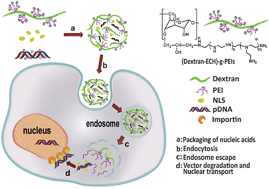
The aim of the present work was to design a targeting polysaccharide-based cationic polymer for gene delivery. PEI is one of the most widely studied cationic polymers in terms of gene delivery, yet its applications are limited by its inherent drawbacks. To address these drawbacks, the grafted low-molecular-weight branched polyethylenimine (PEI) (1800 Da and 800 Da) with the low cytotoxicity and targeting ligand were integrated into a polymeric system based on biodegradable and biocompatible polysaccharide dextran, to afford a highly efficient gene delivery system. The structure of the grafted polymer (Dextran-g-PEI) was confirmed by nuclear magnetic resonance (NMR). DNA complexation resulted in nanoscale and spherical particles (Dextran-g-PEI/DNA), revealed by transmission electron microscopy (TEM). The sizes of the complexes decreased while the zeta potentials increased with the elevation of the w/w ratio. The agarose gel electrophoresis suggested that complexation process alleviates the DNA degradation by DNase I. Very low cytotoxicity was shown on HeLa, 293T and HepG2 cell lines using MTT cell relative viability assay, a red blood cell aggregation assay as well as hemolytic activity determination, whereas transfection efficiency in vitro of Dextran-g-PEI/DNA complexes was observed by green fluorescent protein assay in 293T, HeLa and HepG2 cells. Cy™3 was used as a molecular probe to evaluate the effect of nuclear targeting ligand (PV7) addition on the cellular uptake of complexes. After the intravenous injection of pEGFP to BALB/c mice xenograft models for transfection in vivo, the expression of green fluorescent protein was observed in the transplanted carcinoma and some of the important organs, such as liver, spleen, kidney and lung. To prove the tumor localization of the polyplexes, the in vivo circulation and biodistribution of the polyplexes were monitored by in vivo fluorescent imaging. Thus it was concluded that Dextran-g-PEI is a potential candidate for effective and biocompatible gene delivery systems.

 Please wait while we load your content...
Please wait while we load your content...