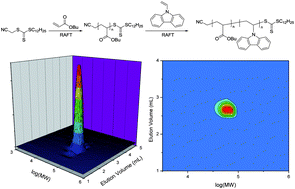
The use of various RAFT agents (ZC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)SR) including dithiobenzoates (Z = Ph), trithiocarbonates (Z = SR′), xanthates (Z = OR′), and conventional and switchable N-aryldithiocarbamates (Z = NR′Ar) in RAFT polymerization of N-vinylcarbazole (NVC) has been explored with a view to establishing which is most effective. Consistent with earlier work, we find that xanthates and N-aryldithiocarbamates give adequate control (dispersities (Đ) < 1.3) while dithiobenzoates give marked retardation. However, contrary to popular belief, we find that the polymerization of NVC is best controlled with trithiocarbonate RAFT agents, which provide both good molecular weight control, very narrow dispersities (Đ < 1.1), and high end-group fidelity. The results demonstrate that NVC has intermediate reactivity, i.e. between that of the traditional more activated (MAMs; styrene, acrylates) and less activated monomers (LAMs; vinyl acetate, N-vinylpyrrolidone). A further key to good control is the selection of RAFT agent R substituent to be both a good leaving group and a good initiating radical. The cyanomethyl group meets these criteria whereas phenylethyl is a poor initiating radical for NVC polymerization. A further demonstration of the intermediate reactivity of NVC and the derived propagating radical was the successful preparation of both poly(n-butyl acrylate)-block-poly(N-vinylcarbazole) and poly(N-vinylcarbazole)-block-poly(n-butyl acrylate) with a trithiocarbonate RAFT agent (the sequence of block synthesis is not important). Two-dimensional, liquid chromatography near critical conditions-gel permeation chromatography (LCCC-GPC) has been applied to demonstrate block purity. The corresponding styrene-based blocks can also be successfully synthesized, however, the reinitiation of NVC polymerization by the polystyryl radical proved to be a constraint on the purity of polystyrene-block-poly(N-vinylcarbazole).
S)SR) including dithiobenzoates (Z = Ph), trithiocarbonates (Z = SR′), xanthates (Z = OR′), and conventional and switchable N-aryldithiocarbamates (Z = NR′Ar) in RAFT polymerization of N-vinylcarbazole (NVC) has been explored with a view to establishing which is most effective. Consistent with earlier work, we find that xanthates and N-aryldithiocarbamates give adequate control (dispersities (Đ) < 1.3) while dithiobenzoates give marked retardation. However, contrary to popular belief, we find that the polymerization of NVC is best controlled with trithiocarbonate RAFT agents, which provide both good molecular weight control, very narrow dispersities (Đ < 1.1), and high end-group fidelity. The results demonstrate that NVC has intermediate reactivity, i.e. between that of the traditional more activated (MAMs; styrene, acrylates) and less activated monomers (LAMs; vinyl acetate, N-vinylpyrrolidone). A further key to good control is the selection of RAFT agent R substituent to be both a good leaving group and a good initiating radical. The cyanomethyl group meets these criteria whereas phenylethyl is a poor initiating radical for NVC polymerization. A further demonstration of the intermediate reactivity of NVC and the derived propagating radical was the successful preparation of both poly(n-butyl acrylate)-block-poly(N-vinylcarbazole) and poly(N-vinylcarbazole)-block-poly(n-butyl acrylate) with a trithiocarbonate RAFT agent (the sequence of block synthesis is not important). Two-dimensional, liquid chromatography near critical conditions-gel permeation chromatography (LCCC-GPC) has been applied to demonstrate block purity. The corresponding styrene-based blocks can also be successfully synthesized, however, the reinitiation of NVC polymerization by the polystyryl radical proved to be a constraint on the purity of polystyrene-block-poly(N-vinylcarbazole).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)SR) including dithiobenzoates (Z = Ph), trithiocarbonates (Z = SR′), xanthates (Z = OR′), and conventional and switchable N-aryldithiocarbamates (Z = NR′Ar) in
S)SR) including dithiobenzoates (Z = Ph), trithiocarbonates (Z = SR′), xanthates (Z = OR′), and conventional and switchable N-aryldithiocarbamates (Z = NR′Ar) in 

 Please wait while we load your content...
Please wait while we load your content...