Higher-oxidation-state iron halides [FeX3 (X = Cl, Br)] were employed in conjunction with a series of ligands, mainly monodentate phosphines and amines, to effect the living radical polymerization of various vinyl monomers such as styrene, methyl methacrylate (MMA), and methyl acrylate (MA). Almost all combinations examined could enable polymerizations in the absence of exogenous reducing agents. However, appropriate combinations of FeX3 and ligands gave rise to polymers in a living manner, with controlled molecular weights and narrow molecular weight distributions (Mw/Mn = 1.1–1.2). Ligand combinations included FeCl3 with PnBu3, PtBu3, or NnBu3 (for styrene); FeCl3 with PtBu3 or NnBu3 (for MMA); and FeBr3 with PPh3 (for MA). Model reactions and spectroscopic analysis suggest that FeCl3 most likely disproportionates into the Fe(III)Cl4− anion and Fe(III)Cl2+ cation in the presence of Lewis base ligands (PR3 and NR3). The latter cationic species, coordinated with the ligand [Fe(III)Cl2(PR3)+ or Fe(II)Cl2(PR3)˙+], acts as the active catalyst. Assistance from the electron-rich ligand allows the catalyst to induce metal-catalyzed living radical polymerization. The Fe(III)-based catalyst could also be easily and almost quantitatively removed from the polymer product simply by washing with aqueous acid to minimize the amount of iron contamination (<5 ppm).
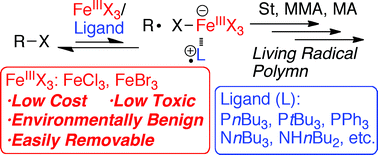
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
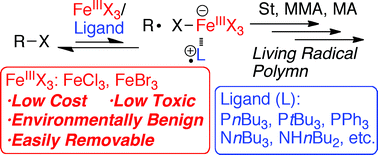

 Please wait while we load your content...
Please wait while we load your content...