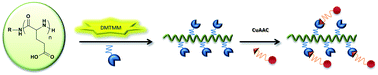
In this article we describe a versatile methodology for the synthesis of polyglutamic acid (PGA) derivatives bearing orthogonal reactive sites. The reactive groups enable selective conjugation chemistry by copper catalyzed azide–alkyne coupling (CuAAC). PGA was derived in aqueous media as well as in organic media using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl morpholinium chloride (DMTTM) salts. The spectra of attached chemical moieties ranges from simple PEGylation with 2,5,8,11,14,17,20-heptaoxadocosan-22-amine (mEG(6)NH2) to the incorporation of propargylamine, 11-azido-3,6,9-trioxaundecan-1-amine (NH2-EG(2)N3), and 20-azido-3,6,9,12,15,18-hexaoxaicosan-1-amine (NH2-EG(6)N3). Herein, it is demonstrated that the degree of functionalization can be easily controlled within this one pot reaction. Additionally, we report conditions for the CuAAC with various PGA derivatives, which can be employed for site-specific conjugation of either hydrophilic or hydrophobic compounds.

 Please wait while we load your content...
Please wait while we load your content...