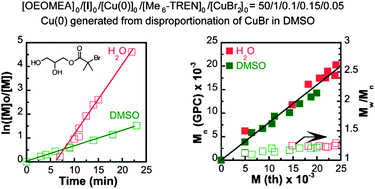
A comparative analysis of the SET-LRP of oligo(ethylene oxide) methyl ether acrylate (OEOMEA) in DMSO and in H2O at 25 °C is reported. Both the catalysis with activated Cu(0) wire/Me6-TREN and with mimics of “nascent” Cu(0) nanoparticles/Me6-TREN resulted in a higher rate of polymerization in water than in DMSO. This result is consistent with the acceleration expected for SET-LRP by a more polar reaction solvent, and with the difference between the equilibrium constants of disproportionation of CuBr in DMSO (Kd = 1.4–4.4) and in water (Kd = 106 to 107), both much higher in the presence of Me6-TREN. The inefficient access of the Cu(0) catalyst to the hydrophobic reactive centers of the monomer and initiator assembled in micellar structures explains the induction time observed in the SET-LRP of OEOMEA in water. This induction period is longer for Cu(0) wire. The use of “nascent” Cu(0) nanoparticles prepared by the disproportionation of CuBr in DMSO, in combination with 5 mol% CuBr2, led to an extremely efficient SET-LRP of OEOMEA in water. This SET-LRP in water is fast and follows first order kinetics to complete monomer conversion with linear dependence of experimental Mn on conversion, and narrow molecular weight distribution. Under the polymerization conditions investigated in both water and DMSO, no reduction in the absorbance of CuBr2/Me6-TREN was observed by online UV-vis spectroscopy. This excludes the formation of CuBr by reduction of CuBr2 by Cu(0) during the SET-LRP in DMSO and in water.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...