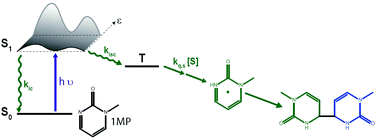
Pyrimidinones are part of the (6-4) photolesions which may be formed from two pyrimidine bases adjacent on a DNA strand. In relation to the secondary photochemistry of the (6-4) lesion, i.e. its transformation into a Dewar valence isomer, photophysical and photochemical properties of 1-methyl-2(1H)-pyrimidinone (1MP) in water, acetonitrile, methanol, and 1,4-dioxane are reported here. As deduced from steady state fluorescence and femtosecond transient absorption spectroscopy the S1 lifetime of 1MP is strongly affected by the solvent. The lifetimes range from 400 ps for water to 40 ps for 1,4-dioxane. Internal conversion (IC) and intersystem crossing (ISC) contribute to the S1 decay. The solvent effect on the IC rate constant is more pronounced than on the ISC constant. The quantum yields for the consumption of 1MP (values for nitrogen purged solvents) are large for methanol (0.35) and 1,4-dioxane (0.24) and small for acetonitrile (0.02) and water (0.003). Hydrogen abstraction from the solvent by the triplet state of 1MP may rationalize this.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...