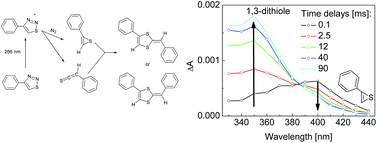
Photochemistry of 4-phenyl-1,2,3-thiadiazole (PT) and 4,5-diphenyl-1,2,3-thiadiazole (DPT) in solution was studied at room temperature using UV-vis and IR transient absorption spectroscopies (λex = 266 nm). Ultrafast techniques show a very fast rise (<0.3 ps) of thiirene and thioketene species, formed from 1,2,3-thiadiazoles in the singlet excited state. The remarkable unimolecular stability of thiirenes in solution is observed. On a millisecond time scale thiirenes with phenyl substituents undergo an intermolecular reaction (dimerization of thiirene–thioketene complexes) leading to 1,3-dithiole derivatives.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...