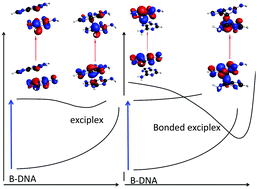
Exciplexes and conical intersections lead to fluorescence quenching in π-stacked dimers of 2-aminopurine with natural purine nucleobases†
Abstract
Fluorescent analogues of the natural DNA bases are useful in the study of nucleic acids’ structure and dynamics.
- This article is part of the themed collection: Interaction of UV radiation with DNA

 Please wait while we load your content...
Please wait while we load your content...