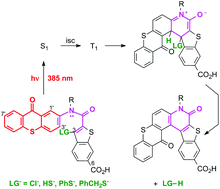
N-(9-Oxothioxanthenyl)benzothiophene carboxamides bearing leaving groups (LG− = Cl−, PhS−, HS−, PhCH2S−) at the C-3 position of the benzothiophene ring system photochemically cyclize with nearly quantitative release of the leaving group, LG−. The LG− photoexpulsions can be conducted with 390 nm light or with a sunlamp. Solubility in 75% aqueous CH3CN is achieved by introducing a carboxylate group at the C-6 position of the benzothiophene ring. The carboxylate and methyl ester derivatives regiospecifically cyclize at the more hindered C-1 position of the thioxanthone ring. Otherwise, the photocyclization favors the C-3 position of the thioxanthone. Quantum yields for reaction are 0.01–0.04, depending on LG− basicity. Electronic structure calculations for the triplet excited state show that excitation transfer occurs from the thioxanthone to the benzothiophene ring. Subsequent cyclization in the triplet excited state is energetically favourable and initially generates the triplet excited state of the zwitterionic species. Expulsion of LG− is thought to occur once this species converts to the closed shell ground state.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...