Multifaceted catalysis approach to nitrile activation: direct synthesis of halogenated allyl amides from allylic alcohols†
Abstract
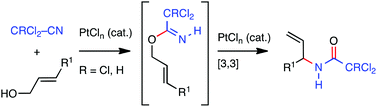
Allyl amides were synthesised from the reaction of allyl alcohols and halogenated nitriles using a platinum multifaceted catalysis approach in which both the nucleophilic addition and subsequent [3,3]-sigmatropic rearrangement steps of the process were catalysed by the same complex. Additionally, 1H/13C{1H} NMR and GC studies provided the first insights into the mechanism of this transformation.

 Please wait while we load your content...
Please wait while we load your content...