Determination of absolute configuration of the phosphonic acid moiety of fosfazinomycins†
Abstract
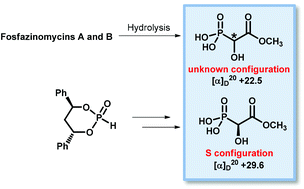
Fosfazinomycins A and B produced by Streptomyces lavendofoliae share the same phosphonate moiety with one chiral centre of unknown configuration which was determined by synthesising both enantiomers of 2-hydroxy-2-phosphonoacetic acid methyl ester. A chiral cyclic phosphite was reacted with methyl glyoxylate in a Pudovik reaction to give a pair of diastereomeric α-hydroxyphosphonates, which were separated by HPLC. The configurations at C-2 were assigned on the basis of single crystal X-ray structure analysis. Deprotection of these diastereomers furnished the enantiomeric α-hydroxyphosphonic acids, of which the (S)-configured had the same sign of optical rotation as the phosphonic acid moiety of the two fosfazinomycins.

 Please wait while we load your content...
Please wait while we load your content...