Metal-free borylative ring-opening of vinyl epoxides and aziridines†
Abstract
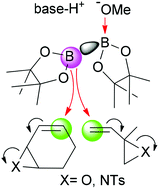
A rational approach towards the borylative ring-opening of vinylepoxides and vinylaziridines, by the in situ formed MeO−→bis(pinacolato)diboron adduct, has been developed. The enhanced nucleophilic character of the Bpin (sp2) moiety from the reagent favours the SN2′ conjugated B addition with the concomitant opening of the epoxide and aziridine rings. The reaction proceeds with total chemoselectivity towards the polyfunctionalised (–OH or –NHTs) allyl boronate. Theoretical calculations have determined the transition states that come from the reaction of the vinylic substrates with the activated MeO−→bis(pinacolato)diboron adduct, and a plausible mechanism for the organocatalytic borylative ring opening reaction has been suggested.

 Please wait while we load your content...
Please wait while we load your content...