Evidence of cation-coordination involvement in directing the regioselective di-inversion reaction of vicinal di-sulfonate esters†
Abstract
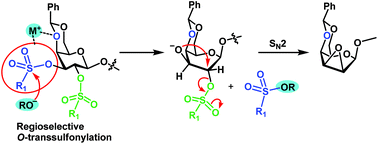
Direct evidence has been obtained to confirm the unusual nucleophilic attack of an alkoxide at the S-center of sp3-hybridized sulfonyl esters. The unusual reaction pathway leads to S–O bond scission which is crucial for the regio- and stereoselective conversion of 2,3-di-O-sulfonates of 4,6-O-benzylidene-β-D-galactopyranosides into β-D-idopyranosides. In addition, strong evidence has been provided to clarify the role of the alkali counter-

 Please wait while we load your content...
Please wait while we load your content...