Evaluating minimalist mimics by exploring key orientations on secondary structures (EKOS)†
Abstract
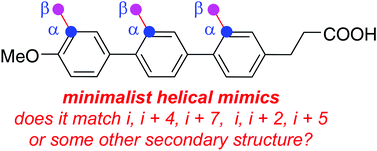
Peptide mimics that display amino acid side-chains on semi-rigid scaffolds (not peptide polyamides) can be referred to as minimalist mimics. Accessible conformations of these scaffolds may overlay with secondary structures giving, for example, “minimalist helical mimics”. It is difficult for researchers who want to apply minimalist mimics to decide which one to use because there is no widely accepted protocol for calibrating how closely these compounds mimic secondary structures. Moreover, it is also difficult for potential practitioners to evaluate which ideal minimalist helical mimics are preferred for a particular set of side-chains. For instance, what mimic presents i, i + 4, i + 7 side-chains in orientations that best resemble an ideal α-helix, and is a different mimic required for a i, i + 3, i + 7 helical combination? This article describes a protocol for fitting each member of an array of accessible scaffold conformations on secondary structures. The protocol involves: (i) use quenched molecular dynamics (QMD) to generate an ensemble consisting of hundreds of accessible, low energy conformers of the mimics; (ii) representation of each of these as a set of Cα and Cβ coordinates corresponding to three amino acid side-chains displayed by the scaffolds; (iii) similar representation of each combination of three side-chains in each ideal secondary structure as a set of Cα and Cβ coordinates corresponding to three amino acid side-chains displayed by the scaffolds; and, (iv) overlay Cα and Cβ coordinates of all the conformers on all the sets of side-chain “triads” in the ideal secondary structures and express the goodness of fit in terms of root mean squared deviation (RMSD, Å) for each overlay. We refer to this process as Exploring Key Orientations on Secondary structures (EKOS). Application of this procedure reveals the relative bias of a scaffold to overlay on different secondary structures, the “side-chain correspondences” (e.g. i, i + 4, i + 7 or i, i + 3, i + 4) of those overlays, and the energy of this state relative to the minimum located. This protocol was tested on some of the most widely cited minimalist α-helical mimics (1–8 in the text). The data obtained indicates several of these compounds preferentially exist in conformations that resemble other secondary structures as well as α-helices, and many of the α-helical conformations have unexpected side-chain correspondences. These observations imply the featured minimalist mimics have more scope for disrupting PPI interfaces than previously anticipated. Finally, the same simulation method was used to match preferred conformations of minimalist mimics with actual protein/peptide structures at interfaces providing quantitative comparisons of predicted fits of the test mimics at protein–protein interaction sites.

 Please wait while we load your content...
Please wait while we load your content...