Abstract
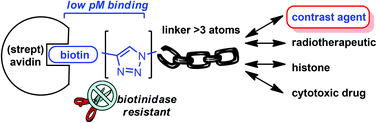
The natural amide bond found in all biotinylated proteins has been replaced with a triazole through CuAAC reaction of an alkynyl biotin derivative. The resultant triazole-linked adducts are shown to be highly resistant to the ubiquitous hydrolytic enzyme biotinidase and to bind avidin with dissociation constants in the low pM range. Application of this strategy to the production of a series of biotinidase-resistant biotin-Gd-DOTA contrast agents is demonstrated.

 Please wait while we load your content...
Please wait while we load your content...