3D structure of a heparin mimetic analogue of a FGF-1 activator. A NMR and molecular modelling study†
Abstract
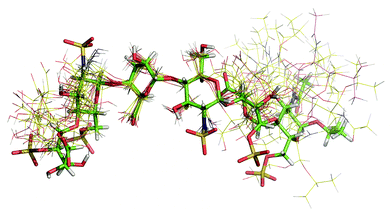
The motional behaviour of heparin oligosaccharides in solution is best described as a top rotor having two perpendicular rotation axes. This prevents an accurate extraction of interprotonic distances by NOESY/ROESY based methods. In this paper, we describe the solution structure of the hexasaccharide 1 calculated from high exactitude distance data obtained from off-resonance ROESY combined with a long MD simulation of 500 ns. In previous studies, we have found that two synthetic hexasaccharides having the sulphate groups directed towards one side of its central plane have an opposite biological activity, while 1 is unable to activate the FGF-1 signalling pathway, the other (2) is even more active than the regular region derived hexasaccharide (3) that mimics the natural active compound, heparin. From the structural analysis it was concluded that 1 has similar three-dimensional characteristics to 2 or 3 and therefore the differences in the activity should be due to the arrangement of the sulphate groups within the hexasaccharidic sequence.

 Please wait while we load your content...
Please wait while we load your content...