Chemoenzymatic synthesis and in situ application of S-adenosyl-l-methionine analogs†
Abstract
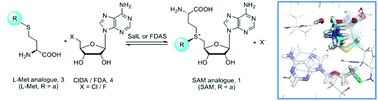
Analogs of S-adenosyl-L-methionine (SAM) are increasingly applied to the methyltransferase (MT) catalysed modification of biomolecules including proteins, nucleic acids, and small molecules. However, SAM and its analogs suffer from an inherent instability, and their chemical synthesis is challenged by low yields and difficulties in stereoisomer isolation and inhibition. Here we report the chemoenzymatic synthesis of a series of SAM analogs using wild-type (wt) and point mutants of two recently identified halogenases, SalL and FDAS. Molecular modelling studies are used to guide the rational design of mutants, and the enzymatic conversion of L-Met and other analogs into SAM analogs is demonstrated. We also apply this in situ enzymatic synthesis to the modification of a small peptide substrate by protein arginine methyltransferase 1 (PRMT1). This technique offers an attractive alternative to chemical synthesis and can be applied in situ to overcome stability and activity issues.

 Please wait while we load your content...
Please wait while we load your content...