Palladium-catalyzed reductive homocoupling of N′-tosyl arylhydrazines†
Abstract
A novel procedure for the preparation of biaryl compounds by Pd-catalyzed homocoupling of N′-tosyl arylhydrazine has been described. N′-Tosyl arylhydrazine, as a readily available and stable coupling partner, demonstrated its generality in the homocoupling reactions. The scope of the reaction and possible mechanism have also been investigated.
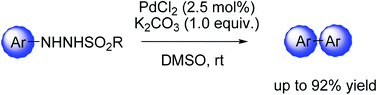

 Please wait while we load your content...
Please wait while we load your content...