Enantioselective total synthesis of macrolide (+)-neopeltolide†
Abstract
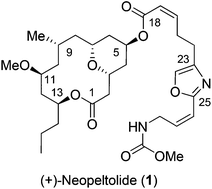
The asymmetric total synthesis of the anti-proliferative macrolide (+)-neopeltolide has been completed. The stereochemically defined trisubstituted tetrahydropyran ring was constructed via a catalytic hetero-Diels–Alder reaction creating two new chiral centers in a highly diastereoselective manner. The other key features of this synthesis included Brown's asymmetric allylation to install the requisite C-11 and C-13 stereocenters. The synthesis of the oxazole side chain consisted of a hydrozirconation of an alkynyl stannane to establish the Z stereochemistry, followed by a palladium catalyzed cross coupling to introduce the desired Z olefin in the oxazole side chain.

 Please wait while we load your content...
Please wait while we load your content...