Development of bis-unsaturated ester aldehydes as amino-glue probes: sequential double azaelectrocyclization as a promising strategy for bioconjugation†
Abstract
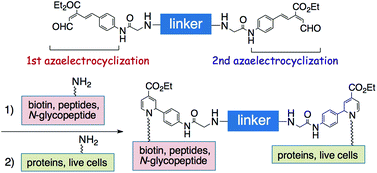
The unsaturated ester aldehyde, (E)-3-alkoxycarbonyl-5-phenyl-2,4-dienal, was efficiently dimerized by applying the strain-promoted double-click reaction with sym-dibenzo-1,5-cyclooctadiene-3,7-diyne. The resulting dimerized probe was sequentially reacted first with one peptide molecule and then with a protein or the amino groups on the surface of a live cell through double azaelectrocyclization to achieve highly efficient bioconjugation.

 Please wait while we load your content...
Please wait while we load your content...