Theoretical study on the mechanism and stereochemistry of the cinchona–thiourea organocatalytic hydrophosphonylation of an α-ketoester†
Abstract
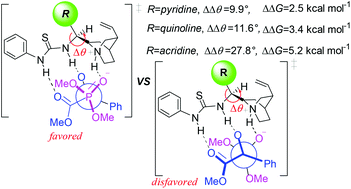
The mechanism and stereochemistry of the hydrophosphonylation of an α-ketoester with dimethylphosphonate (DMHP) catalyzed by a thiourea–cinchona organocatalyst have been studied by the ONIOM method. The calculations show that the catalytic cycle is a three-step process, including the deprotonation of DMHP, C–P bond formation via nucleophilic addition and proton transfer with the regeneration of the catalyst. The deprotonation of DMHP mediated by the basicity of the quinuclidine nitrogen atom is the rate-determining step for the entire reaction. The activation of the α-ketoester by the thiourea or protonated cinchona moiety of the bifunctional catalyst is comparatively investigated, and the former is energy-preferred. AIM combined with NBO analysis indicate that the multiple hydrogen bonds play essential roles in activating substrates, facilitating charge transfer and stabilizing transition states and intermediates. The stereochemistry of the reaction is controlled by the C–P bond formation step and originated from the chiral induction of the multiple hydrogen-bonding interactions. The bulkier substituent groups on the chiral scaffold of the catalyst may increase rigidity of the catalyst and the asymmetric induction to the substrates. The calculations predict that alkyl substituted α-ketoesters might also be converted to chiral α-hydroxyl phosphonates with high enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...