Experimental evidence of a cyclopropylcarbinyl conjugative electronic effect†
Abstract
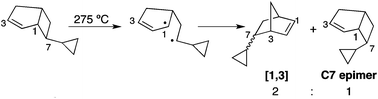
Bicyclo[3.2.0]hept-2-enes undergo thermal rearrangement to norbornenes via diradical transition structures. The synthesis of exo-7-cyclopropylbicyclo[3.2.0]hept-2-ene has been achieved by cycloaddition of cyclopentadiene and cyclopropylketene, generated by treatment of cyclopropylacetyl chloride with triethylamine. A comparison of the cyclopropyl substituent effect with that of other C7 substituents provides experimental evidence of an electron-donating conjugative effect on the transient diradical transition structure in the thermal reaction of exo-7-cyclopropylbicyclo[3.2.0]hept-2-ene.

 Please wait while we load your content...
Please wait while we load your content...