Pd(ii)–SDP-catalyzed enantioselective 5-exo-digcyclization of γ-alkynoic acids: application to the synthesis of functionalized dihydofuran-2(3H)-ones containing a chiral quaternary carbon center†
Abstract
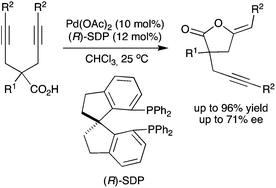
The Pd(II)–SDP-catalyzed first enantioselective intramolecular

 Please wait while we load your content...
Please wait while we load your content...